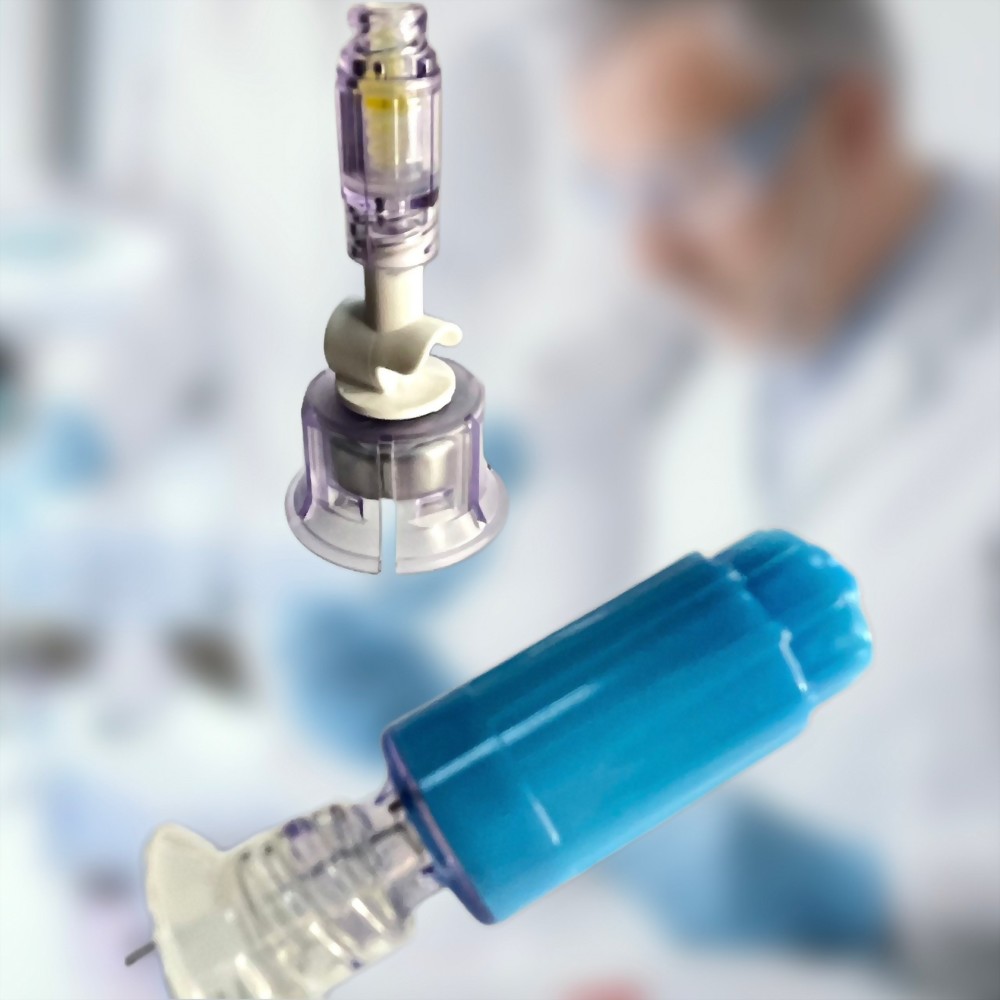
Closed Male Luer 12C009
Closed Male Luer 12C009
Description Comply with ISO 80369-7 standards, universal design.
Simple mechanism design
Easy to clean swabable surface.
100 PSI pressure compatibility
Pass biocompatibility test
Patented model designs with multiple countries' patents.
Simple mechanism design
Easy to clean swabable surface.
100 PSI pressure compatibility
Pass biocompatibility test
Patented model designs with multiple countries' patents.
Model :
12C009
- 12C009
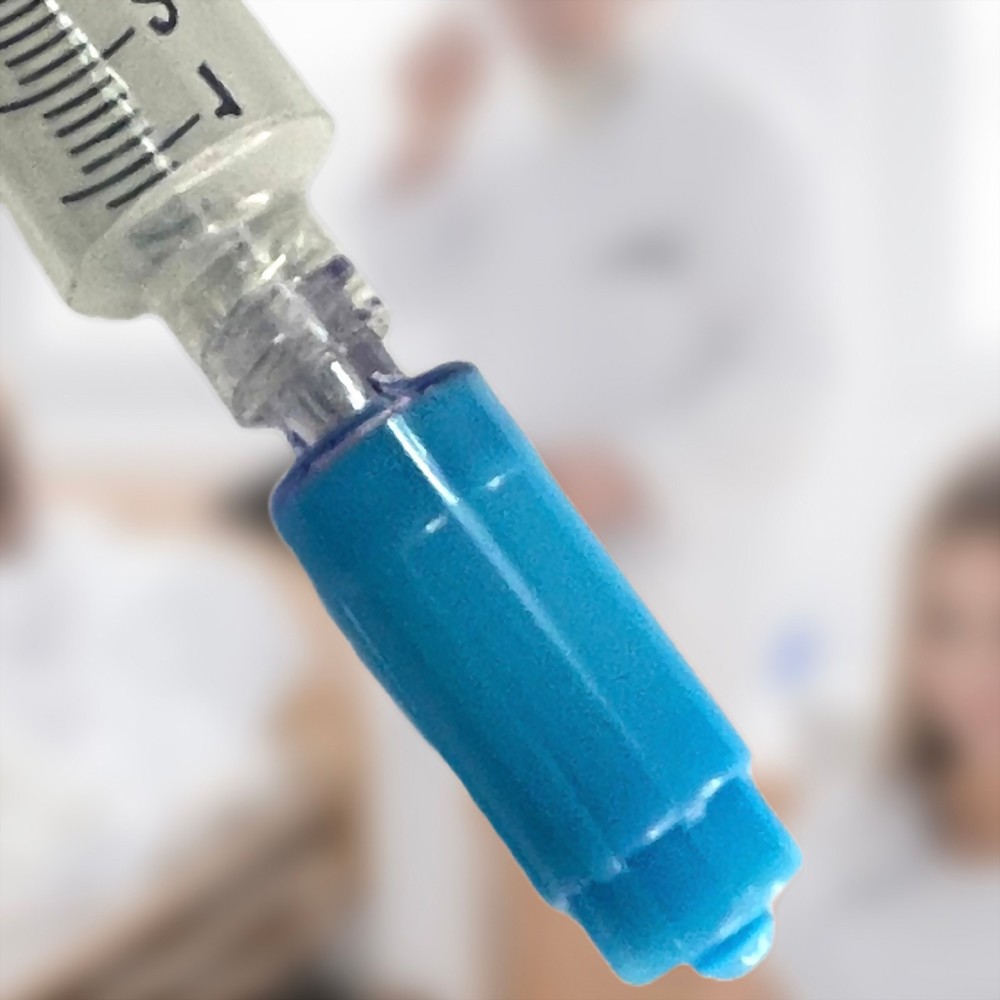
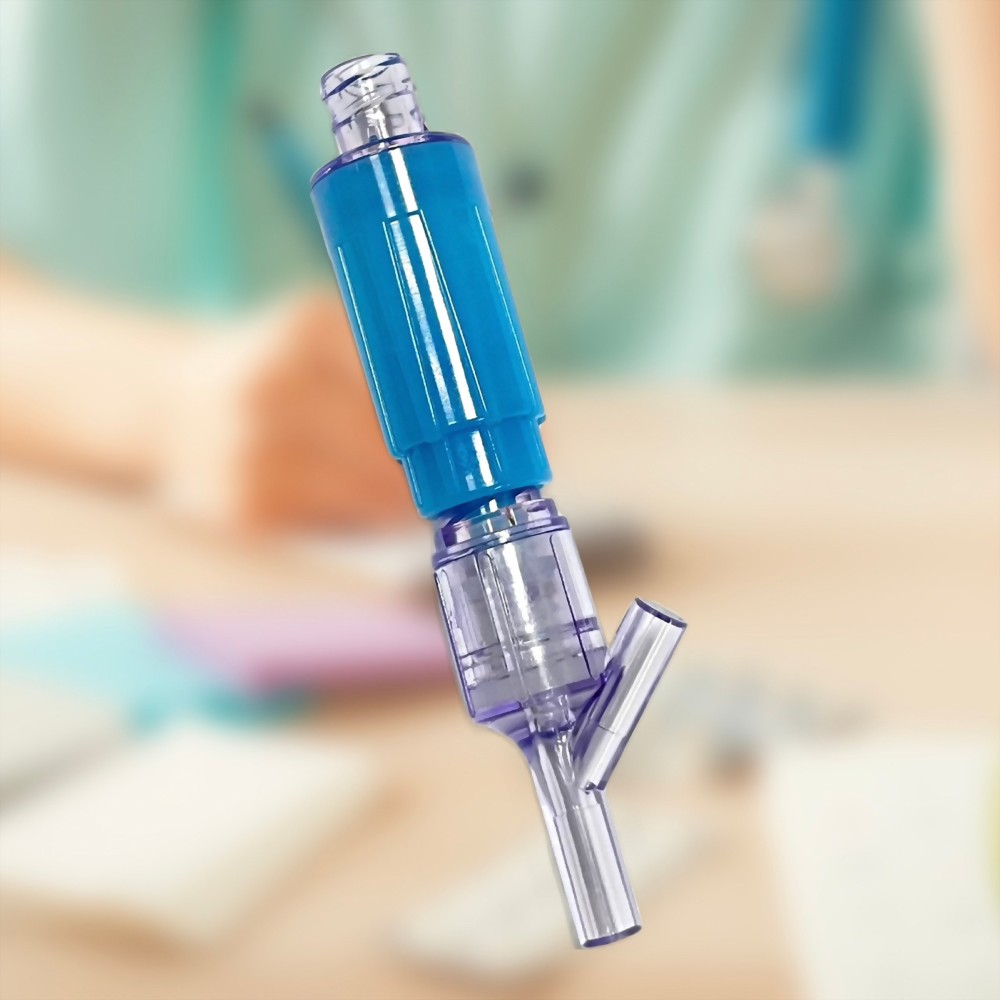
- 12C009 is a closed male luer designed for Gen2 products and other types of NFC (needle-free connector), facilitating easy connection and drug transfer.
- It features a passive closing function, allowing the connection to open only when connected to female luer lock connectors.
- Upon disconnection, it incorporates a function to absorb any residual drugs, enhancing safety and minimizing waste.
- Description Comply with ISO 80369-7 standards, universal design.
- Simple mechanism design
- Easy to clean swabable surface.
- 100 PSI pressure compatibility
- Pass biocompatibility test
- Patented model designs with multiple countries' patents.
- Volume
- Total length*maximum diameter 36.3mm * 12.75mm
- Number of parts
- Assembled with 4 components
- Surface residue
- Connect with Gen2 connector P/N:12C007 or other brands
- Thread matching
- Conform to ISO 594
- Pressure Test
- The maximum operating pressure to bear on 200psi
- Residual
- The internal residual of after the operation is completed0.24ml
- Flow Rate
- 80ml/min, higher than the patient's head more than 90CM.
High-Risk Procedures Demanding Rigorous Standards:
- Chemotherapy infusion and drug preparation are high-risk medical procedures due to the toxicity of chemotherapy drugs.
- Strict adherence to safety and quality standards, such as USP 797, is essential to protect patients and healthcare professionals.
- Sets comprehensive guidelines for compounding and infusion processes.
- Focuses on ensuring safety, quality, and hygiene.
- Covers facility requirements, staff protocols, and risk mitigation measures.
- Clean, orderly, and well-maintained environment.
- Easy to clean and disinfect.
- Proper equipment and supplies.
- Measures to prevent contamination (dust, splashes, etc.).
- Sterile technique usage.
- Prevention of cross-contamination.
- Accurate labeling of medications.
- Understanding of high-risk procedures.
- Healthcare institutions should comply with local and international standards, including potential additional requirements in their region.
- Closed systems that transfer chemotherapy drugs from original packaging to infusion components.
- Help reduce contamination and risks during compounding and infusion.
- Aligned with USP 797 principles.
- USP 797 provides guidelines but allows flexibility for healthcare institutions to choose products that meet their specific needs and standards.
- Offers products, including closed male luers and other components, designed to protect healthcare professionals during chemotherapy procedures.
- Products align with USP 797 guidelines.
- Contribute to maintaining a sterile and safe environment.
- Regular staff training and competency assessment.
- Comprehensive quality management systems and audits.
- Use of products and equipment that meet USP 797 standards.
- Robust documentation and reporting systems.