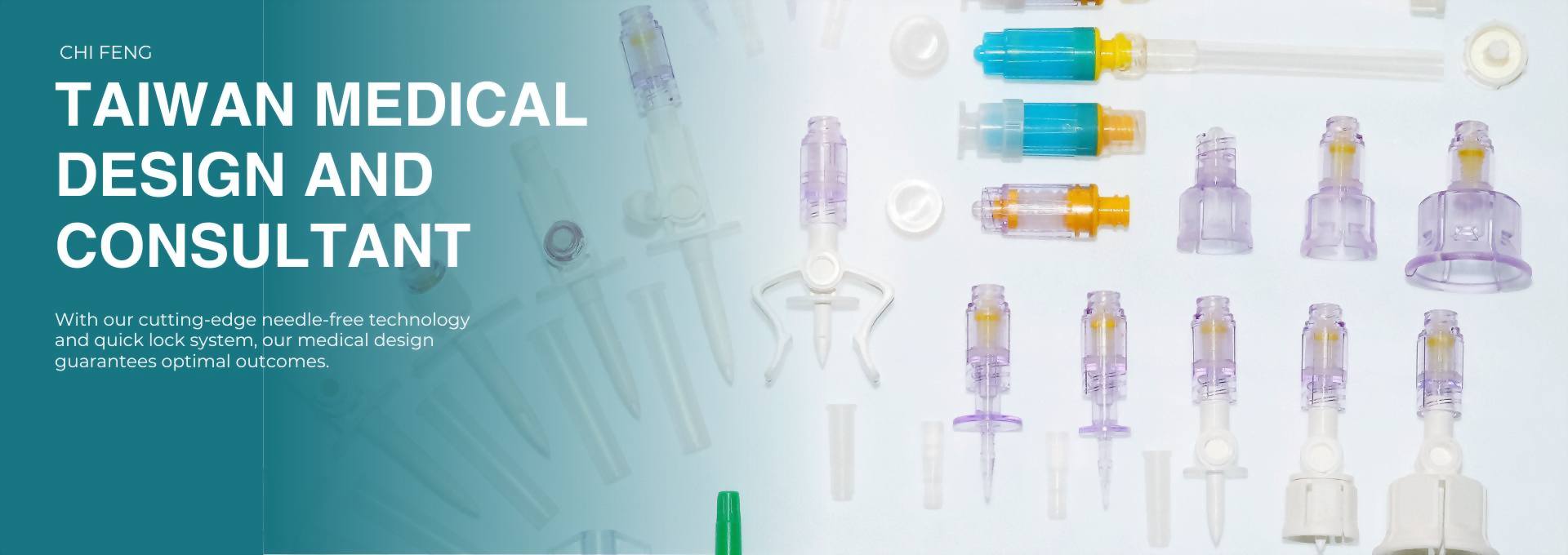
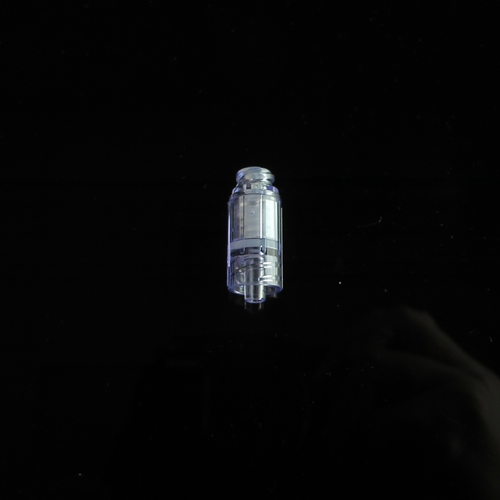
Gen2 Needle Free Connector 14C007
Gen2 Needle Free Connector 14C007
Injection site
Color : Transparent
Material : PC + MABS
Application : for CSTD with P/N: 12C009 CML or for infusion type
Color : Transparent
Material : PC + MABS
Application : for CSTD with P/N: 12C009 CML or for infusion type
Model :
14C007
- 14C007
- Injection site
- Color : Transparent
- Material : PC + MABS
- Application : for infusion sets, IV line...etc.
- Materials - Medical grade polycarbonate and medical grade silicone, biocompatibility test pass.
- Pressure type - Neutral pressure.
- Functional activation - Up to 200 actuations.
- Flow rate - 120 ~ 145 ml / min (at 35.4 inch head height).
- Residual volume - 0.016ml
- Reflux Volume - 0.01ml
- Flush Volume - Minimal dead space (also referred to as residual volume) of 0.016 mL allows for lower flush volumes.
- Disconnect force - <1kg
- Pressure leakage - Up to 1.5kg/cm2 of air (Disconnection)
- Back Pressure40~70psi
- Pressure Maximum:140~300psi
- Alcohol - Compatible : Yes.
- Lipid - Compatible : Yes.
- Development and Production:
- Needleless infusion devices are developed and produced by CHI-FENG, acquiring patents from multiple countries.
- Gen 2 Needleless Infusion Series:
- Utilizes a neutral pressure design to minimize discomfort for patients during needle insertion and removal, reducing pressure-related pain.
- Excludes metal components to prevent malfunctions and MRI interference.
- Fitting part follows ISO 594 standards, passing necessary tests for compatibility with a wide range of infusion products.
- Housing and septum material use medical-grade polycarbonate and silicone, ensuring visibility of the fluid path, passing bio-compatibility tests, and resisting lipids and alcohol.
- Silicone seal can be activated up to 200 times, and the swabable surface is sealed around the top of the housing, creating an initial microbial barrier.
- Fluorescein Dye Test:
- In the fluorescein dye test, no residual dye is observed on the swabable surface after disconnecting and swabbing.
- High-Pressure Infusion Capability:
- In high-pressure infusion therapy, #16C002 is available for the Gen 2 needleless series, capable of withstanding pressures up to 22.8kg/cm2 (325PSI).
- Comply with ISO 594 standards
- Easy to clean swabable surface
- Neutral pressure, reduce the pain of insert and pull out the needle
- Low residual, only 0.016ml priming volume when disconnected
- Biocompatibility test pass
- Easy to visualize the fluid path
- Small size, acquired patents from multiple countries