Needle Free Connectors
Needle Free Connectors
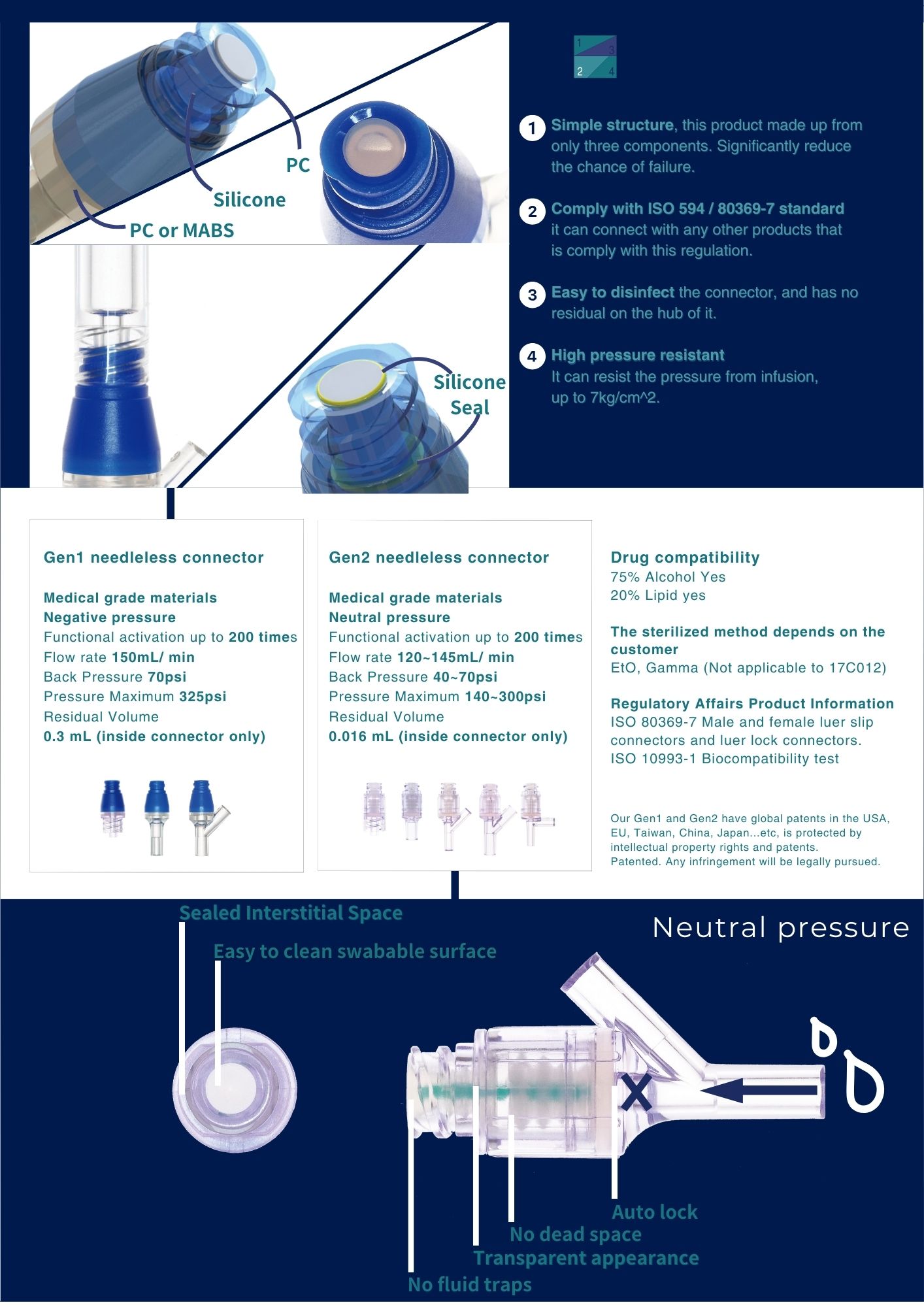
- Complies with ISO 594/ ISO 80369-7 standards
- Features a simple mechanism design
- Easy-to-clean swabable surface
- High-pressure compatibility
- Successfully passes biocompatibility tests
- Boasts patented model designs with multiple countries' patents
Needleless Infusion Devices by CHI-FENG:
- Developed and produced by CHI-FENG
- Acquired patents from multiple countries
- Gen 1 needleless series contains no metal material
- Fitting part follows ISO 594/ ISO 80369-7 standards and passes relevant tests
- Compatible with infusion products adhering to ISO 594 / ISO 80369-7 standards
- Minimizes application of force for linking to prevent self-dropping of the fitting part
- Housing and septum materials use medical-grade polycarbonate
- Passed medical-grade silicone and bio-compatibility tests
- Resistant to lipids and alcohol
- Housing structure passes blood compatibility test, reducing hemolysis
- Silicone seal can withstand up to 200 actuations
- Swabable surface sealed around the top of the housing for microbial barrier protection
- Fluorescein dye test shows no residual dye on the swabable surface after disconnecting
- Suitable for high-pressure infusion therapy
- Can resist a maximum pressure of 7kg/cm2 when the insert part is not connected
- When connected, can resist a maximum pressure of 24kg/cm2 (350PSI)